What is CPAM?
A congenital pulmonary airway malformation (CPAM), also known as congenital cystic adenomatoid malformation (CCAM), is a cystic piece of abnormal lung tissue that does not work like normal lung tissue. It usually replaces one part (lobe) of the lung. CPAMs occur with equal frequency in both lungs. We do not know what causes one part of the lung to develop abnormally, but we know that cystic tissue involved will never function as normal lung tissue.
There are several types of cystic lung disease including CPAM, pulmonary sequestration, and defects that are a mixture of these two. The abnormal piece of lung can be microcystic (many small cysts) or macrocystic (several large cysts). Pulmonary sequestrations are distinguished from CPAMs by a blood vessel that comes directly from the main artery (aorta) and into the lung mass. The type is not as important as the size and how much it enlarges during the pregnancy.
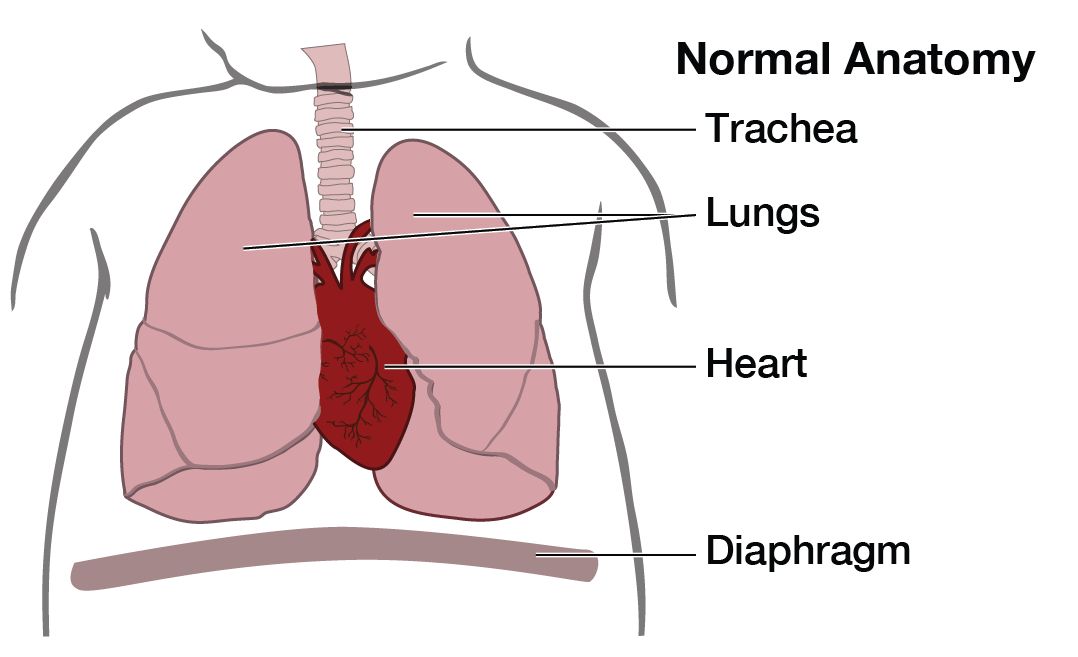
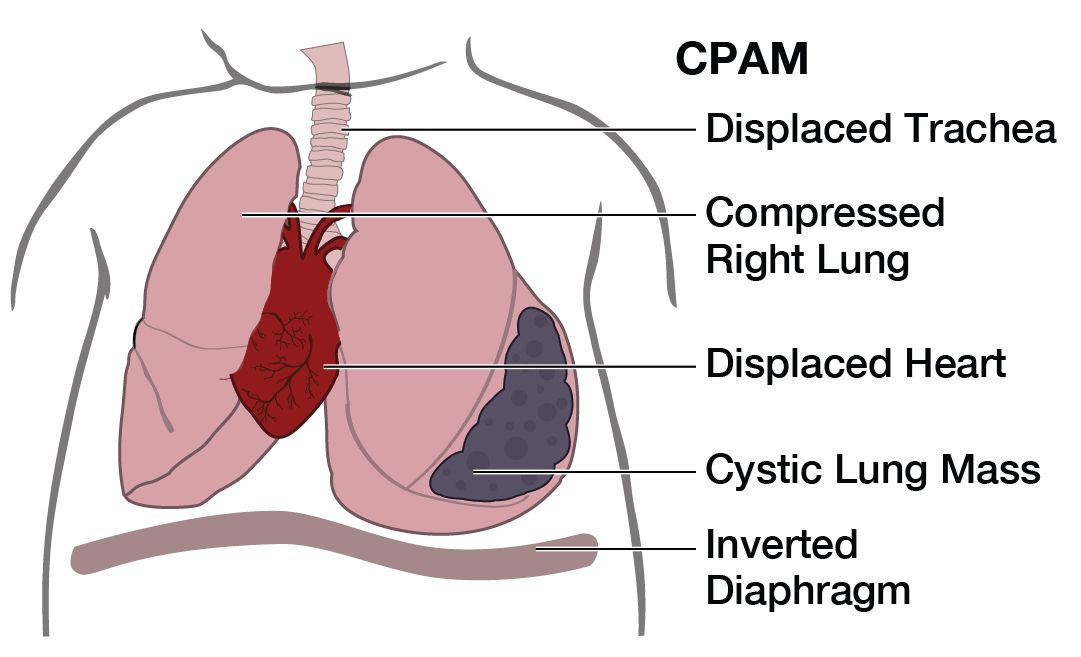
What is the outcome for a fetus with CPAM?
CPAMs vary in size at presentation and can change dramatically throughout the pregnancy. The diagnosis is made by prenatal ultrasonographic findings of an echogenic (bright) mass appearing in the chest of the fetus. Other ultrasound findings may include displacement of the heart from its normal position, a flat or everted (pushed downward) diaphragm, or the absence of visible lung tissue.
The majority of these fetuses have a very good outcome. The mass may grow with the fetus and appear quite large, but not cause trouble because the fetus is growing rapidly and there remains enough room for the normal part of the lung to grow. The mass may remain the same size, but because the fetus is growing rapidly, the abnormal piece of lung becomes relatively small. The mass may shrink in size or even disappear before birth. In all these cases, the outlook for a normal life is excellent.
For a minority of patients (less than 10%), the mass grows so large that it becomes life-threatening to the fetus. These fetuses develop hydrops—accumulation of extra fluid in the skin, scalp, chest, or abdomen that reflect severe heart failure. The mass can grow so large that it limits the growth of the lungs resulting in pulmonary hypoplasia, or small lungs. The mass can also push on the heart and the esophagus of the fetus, putting an extra workload on the heart and preventing the fetus from swallowing amniotic fluid. Often the first sign of a problem during the pregnancy is a mother who measures too big for her due date because there is too much amniotic fluid.
Those who do not have hydrops when the lesion is first detected must be closely followed by doing ultrasounds at regular intervals to look for the development of hydrops. If hydrops does not develop, we continue a ‘wait-and-see’ attitude with close follow-up. Many of these lesions will begin to decrease in size by 28 weeks of gestation and can be safely treated after birth. Infants who do not have respiratory symptoms at birth can be followed by a pediatric surgeon at a tertiary perinatal center.
Fetuses with very large rapidly growing lesions (usually between 20 and 26 weeks’ gestation) may develop hydrops (fetal heart failure) and become very ill with a severe risk of death. Fetuses with CPAM and hydrops will generally not survive unless treated. In addition, severe hydrops can be a threat to the mother if the mirror syndrome develops, i.e., the mother “mirrors” the fetus’s illness, developing high blood pressure and fluid retention (edema).
Maternal Mirror Syndrome
In cases with extreme fetal hydrops, the mother may be at risk for maternal mirror syndrome, which is a condition where the mother's condition mimics that of the sick fetus. Because of a hyperdynamic cardiovascular state, the mother develops symptoms that are similar to pre-eclampsia and may include vomiting, hypertension, peripheral edema (swelling of her hands or feet), protein in her urine and pulmonary edema (fluid in her lungs). Despite resection of the anomaly, maternal mirror syndrome may still occur.
How serious is my fetus’s condition?
In order to determine the severity of your fetus's condition it is important to gather information from a variety of tests and determine if there are any additional problems. These tests along with expert guidance are important for you to make the best decision about the proper treatment.
This includes:
- The type of defect—distinguishing it from other similar appearing problems.
- The severity of the defect—is your fetus’s defect mild or severe.
- Associated defects—is there another problem or a cluster of problems (syndrome).
Amniocentesis may be advised for chromosome testing. Sonography is the best imaging tool. Magnetic resonance imaging may be necessary in some cases. Many problems are first detected during routine screening procedures performed in your doctor's office (amniocentesis, maternal serum screening, routine sonography), but assessment of complex fetal problems requires a tertiary perinatal/neonatal center with experience managing complex and rare fetal problems. We can work with your doctor to find a center convenient for you.
What are my choices during this pregnancy?
Most fetuses with Congenital Cystic Adenomatoid Malformation do not require intervention during the pregnancy and will do well with management after birth. This treatment usually involves surgery at around 6–9 months after birth to remove the abnormal piece of lung.
Fetuses with large CPAM on prenatal ultrasound are initially followed closely by our center, usually once per week, but sometimes 2-3 times/week. Fetuses that get sick exhibit hydrops fetalis on ultrasound. Fluid around the heart, lungs, intestine as well as thickening of the skin or placenta, and polyhydramnios are signs of hydrops fetalis. When hydrops fetalis develops in fetuses with CPAM, nearly 100% of fetuses will die without treatment. In fetuses with CPAM's without hydrops, nearly 100% survive.
Minimally Invasive Fetal Surgery: Thoracoamniotic Shunt
At our fetal treatment center, we pioneered fetal surgical techniques for treatment of fetuses with CPAM and hydrops. Those fetuses that have macrocystic (large sacs) CPAM can have a procedure known as a thoracoamniotic shunt placement. This procedures drains fluid out of the large sacs, decreasing the size of the CPAM. The thoracoamniotic shunt procedure is performed through a needle, and the mother does not need an incision for this minimally invasive procedure.
Surgical Treatment at Birth: EXIT procedure
A few fetuses with very large lesions that will make resuscitation after delivery dangerous will need special management at birth: a specialized delivery called the EXIT (ex utero intrapartum treatment) procedure. We developed the EXIT procedure for management of fetal airway emergencies, but it can be adapted to allow surgical resection of the CPAM while the baby remains attached to the placenta. The EXIT procedure can also be used to facilitate immediate support using extracorporeal membrane oxygenation (ECMO).
Prenatal Steroid Treatment
A new treatment for fetuses with microcystic CPAMs that have hydrops has emerged here at UCSF. Our group has discovered that steroids (betamethasone), which are commonly given in pregnancy, may help fetuses with large CPAMs to either prevent or reverse hydrops fetalis. Our study published in 2003 showed the potential efficacy of steroids for treatment in these situations. Groups subsequently at the Children's Hospital of Philadelphia and the University of Cincinnati have found similar promising results.
We utilize ultrasound measurements to determine whether to give steroids to the mother. The size of the mass, measured by chest volume ratio (CVR) must be greater than 1.6. At that time 2 doses of steroids 24 hours apart are given to the mother.
Open Fetal Surgical Resection of the CPAM
In the rare instance steroid therapy is not effective, large CPAMs causing hydrops may be amenable to fetal surgical resection. Only the most severely affected fetuses are candidates, non-responsive to steroids, for this intervention. In those with a microcystic CPAM (without a large sac), open fetal surgery has been the only option for a chance at survival. Fetal surgery is only offered when there is evidence of hydrops (heart failure) in the fetus. Women with signs of pre-eclampsia (high blood pressure, protein in the urine) have mirror syndrome and are not candidates for fetal intervention.
Fetal surgical resection to remove the abnormal lobe of the lung (lobectomy) is the same surgery that is done after birth. Open fetal surgery is similar to a c-section without clamping the umbilical cord. The fetus is returned into the womb after surgery to continue the pregnancy.
While this procedure gives hope for survival (about 50% of babies survive) mothers must undergo a significant procedure, and the risk for preterm labor after fetal surgery is high. Many of these babies, because of the preterm labor of the mother are born as premature infants and have the risks associated with prematurity.
What will happen after birth?
Fetuses with small or moderate-sized CPAMs that do not change much during pregnancy can be successfully managed after a normal birth. They usually do not have any difficulty during pregnancy or any trouble breathing at the time of birth. If imaging studies (X-ray or CT scan) show a significant CPAM mass, or if the baby has any difficulty breathing, the CPAM should be surgically removed to prevent the development of infection or possible cancer later in life. This surgery is quite safe even in the first year of life and does not compromise lung function or the normal development of the baby. These children will have perfectly normal lung function.
If the mass is very small and the baby has no difficulty breathing, the baby can go home. If the mass remains very small on CT scan at 3-6 months, surgery is not necessary. However, if the CPAM is larger or the baby develops any symptoms (recurrent respiratory infections), then the CPAM should be removed.
Support Groups & Other Resources
- March of Dimes — Researchers, volunteers, educators, outreach workers and advocates working together to give all babies a fighting chance
- Birth Defect Research for Children — a parent networking service that connects families who have children with the same birth defects
- Kids Health — doctor-approved health information about children from before birth through adolescence
- CDC - Birth Defects — Dept. of Health & Human Services, Centers for Disease Control and Prevention
- NIH - Office of Rare Diseases — National Inst. of Health - Office of Rare Diseases
- North American Fetal Therapy Network — NAFTNet (the North American Fetal Therapy Network) is a voluntary association of medical centers in the United States and Canada with established expertise in fetal surgery and other forms of multidisciplinary care for complex disorders of the fetus.